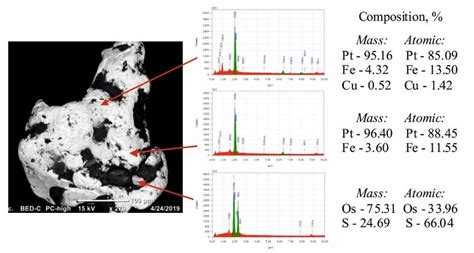
Title: Platinum Grain Structure: Unveiling the Inner Workings of this Noble Metal
Introduction:

Platinum, known for its exquisite luster and remarkable durability, has long been prized across various industries. This precious metal is not only used in jewelry but also serves as a crucial component in many technological applications. One of the most fascinating aspects of platinum is its unique grain structure, which plays a vital role in its properties and performance. In this article, we will delve into the intricate details of the platinum grain structure and its significance in the realm of metallurgy and technology.
Understanding the Grain Structure:
Grain structure refers to the arrangement of crystals within a metal. In the case of platinum, its grain structure is characterized by a face-centered cubic (FCC) lattice, which is one of the most stable crystal structures. This arrangement of atoms creates a strong, interconnected network that contributes to platinum’s exceptional properties.
The Formation of Platinum Grains:
The process of grain formation in platinum involves the growth and development of crystal nuclei within the metal. Initially, these nuclei are tiny and can be influenced by various factors, such as temperature, pressure, and the presence of impurities. Over time, these nuclei grow and merge, resulting in the formation of larger grains. The grain boundaries, which separate individual grains, also play a crucial role in the overall structure and properties of platinum.
Significance of the Platinum Grain Structure:
1. Ductility and Malleability: The FCC grain structure of platinum enables it to be highly ductile and malleable. This property allows platinum to be shaped into various forms, from delicate jewelry to intricate electronic components.
2. Thermal Conductivity: Platinum possesses excellent thermal conductivity, which is essential in many applications, such as catalysis and thermocouples. The grain structure contributes to this property by providing a high degree of atomic mobility, allowing for efficient heat transfer.
3. Resistance to Corrosion: The stable grain structure of platinum makes it highly resistant to corrosion, which is why it is commonly used in catalytic converters and chemical processing industries.
4. Stability at High Temperatures: Platinum maintains its structural integrity at high temperatures, making it suitable for applications that require heat resistance, such as in aerospace and power generation industries.
Influence of Grain Size on Platinum Properties:
The grain size of platinum can be manipulated through various methods, such as annealing or cold working. By altering the grain size, we can fine-tune the metal’s properties to meet specific application requirements.
Conclusion:
The platinum grain structure is a crucial aspect of this noble metal, contributing to its exceptional properties and widespread use in various industries. Understanding the intricate details of its grain structure allows scientists and engineers to harness the full potential of platinum, ensuring its continued relevance in the modern world.