# Electroplating Current Density: A Key Factor in Plating Quality
Electroplating is a widely used industrial process for applying a thin layer of metal to a substrate. This process finds applications in various industries, including automotive, electronics, and aerospace. One of the most critical parameters in electroplating is current density, which plays a pivotal role in determining the quality of the plated layer. This article delves into the concept of electroplating current density and its significance in the electroplating process.

## What is Electroplating Current Density?
Electroplating current density refers to the amount of electric current passing through a given area of the electrolyte per unit time. It is measured in amperes per square meter (A/m²). The current density in an electroplating bath is crucial as it influences several aspects of the electroplating process, such as the thickness of the plated layer, the quality of the finish, and the uniformity of the deposit.
## The Relationship Between Current Density and Plating Thickness
The thickness of the plated layer is directly proportional to the current density. As the current density increases, the thickness of the plated layer also increases. This relationship is described by Faraday’s laws of electrolysis, which state that the mass of a substance deposited at an electrode is proportional to the quantity of electricity that passes through the electrolyte.
However, it is important to note that increasing current density beyond a certain point can lead to overplating, which results in an uneven finish and increased porosity in the plated layer. Therefore, it is essential to optimize the current density to achieve the desired thickness while maintaining the quality of the plated layer.
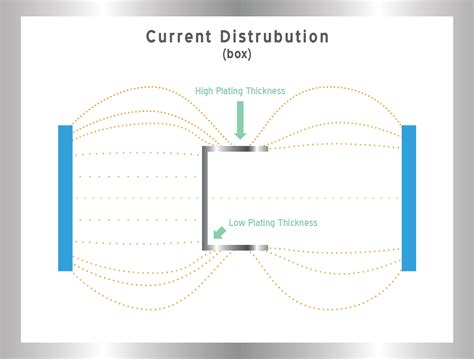
## Current Density and Plating Quality
The quality of the plated layer is significantly influenced by the current density. Some of the key aspects affected by current density include:
1. Uniformity: A higher current density can lead to non-uniform plating, especially in complex shapes. This is due to the uneven distribution of current in the electrolyte, which results in variations in thickness and finish.
2. Porosity: Overplating caused by excessive current density can lead to porosity in the plated layer. Porous layers are susceptible to corrosion and can reduce the overall durability of the plated part.
3. Adhesion: The adhesion between the plated layer and the substrate can be affected by current density. Excessive current density can cause a weak bond, which may lead to flaking and delamination.
4. Surface finish: The surface finish of the plated layer is influenced by the current density. A higher current density can result in a rougher finish, while a lower current density can lead to a smoother finish.
## Optimizing Current Density
Optimizing the current density is crucial for achieving high-quality electroplating. Here are some factors to consider when determining the optimal current density:
1. Plating process: The type of electroplating process (e.g., acidic, alkaline, or neutral) can influence the optimal current density.
2. Plating material: Different metals require different current densities for achieving the desired plated layer thickness and quality.
3. Bath composition: The concentration of the electrolyte and the presence of additives can affect the optimal current density.
4. Temperature: The temperature of the electrolyte can impact the current density and, consequently, the plated layer quality.
By carefully considering these factors and conducting experiments to determine the optimal current density, electroplaters can achieve high-quality plated layers with excellent uniformity, adhesion, and surface finish.